Techno-economic analysis of a sustainable process for converting CO2 and H2O to feedstock for fuels and chemicals†
Abstract
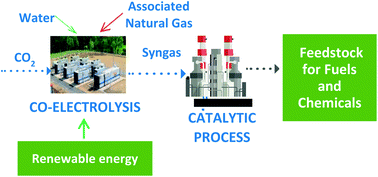
Environmentally friendly and economically competitive production of renewable fuels and chemicals from CO2 and H2O, harnessing renewable electricity, is a key component in the solution to climate change. Integration of highly efficient solid oxide electrolysis cells (SOECs) to convert CO2 and H2O to syngas – a mixture of H2 and CO at a molar ratio of 0.7, with a high-performance process for converting syngas to liquid feedstock for fuels and chemicals is described in this article. A novel configuration of SOECs provides a low-cost solution to produce high syngas production rates at low cell degradation. Integration of natural gas in the process directly to the anode further improves the economics and energy management. In the fuel synthesis reactor, CO conversion was 82% while C5+ selectivity of the combined CO conversion and oligomerization processes was 79%. Stable operation for 4000 h was demonstrated. The overall energy efficiency was 67%. The economics of the process depends mainly on the cost of energy and the size of the plant.


 Please wait while we load your content...
Please wait while we load your content...