Synthesis of a heterobimetallic actinide nitride and an analysis of its bonding†
Abstract
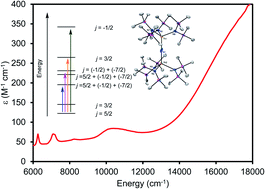
Reaction of [K(DME)][Th{N(R)(SiMe2CH2)}2(NR2)] (R = SiMe3) with 1 equiv. of [U(NR2)3(NH2)] (1) in THF, in the presence of 18-crown-6, results in formation of a bridged uranium–thorium nitride complex, [K(18-crown-6)(THF)2][(NR2)3UIV(μ-N)ThIV(NR2)3] (2), which can be isolated in 48% yield after work-up. Complex 2 is the first isolable molecular mixed-actinide nitride complex. Also formed in the reaction is the methylene-bridged mixed-actinide nitride, [K(18-crown-6)][K(18-crown-6)(Et2O)2][(NR2)2U(μ-N)(μ–κ2-C,N–CH2SiMe2NR)Th(NR2)2]2 (3), which can be isolated in 34% yield after work-up. Complex 3 is likely generated by deprotonation of a methyl group in 2 by [NR2]−, yielding the new μ-CH2 moiety and HNR2. Reaction of 2 with 0.5 equiv. of I2 results in formation of a UV/ThIV bridged nitride, [(NR2)3UV(μ-N)ThIV(NR2)3] (4), which can be isolated in 42% yield after work-up. The electronic structure of 4 was analyzed with EPR spectroscopy, SQUID magnetometry, and NIR-visible spectroscopy. This analysis demonstrated that the energies of 5f orbitals of 4 are largely determined by the strong ligand field exerted by the nitride ligand.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...