When SF5 outplays CF3: effects of pentafluorosulfanyl decorated scorpionates on copper†
Abstract
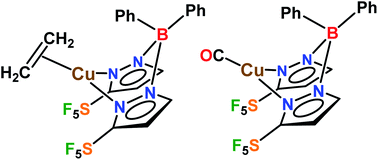
Polyfluorinated, electron-withdrawing, and sterically demanding supporting ligands are of significant value in chemistry. Here we report the assembly and use of a bis(pyrazolyl)borate, [Ph2B(3-(SF5)Pz)2]− that combines all such features, and involves underutilized pentafluorosulfanyl substituents. The ethylene and carbonyl chemistry of copper(I) supported by [Ph2B(3-(SF5)Pz)2]−, a comparison to the trifluoromethylated counterparts involving [Ph2B(3-(CF3)Pz)2]−, as well as copper catalyzed cyclopropanation of styrene with ethyl diazoacetate and CF3CHN2 are presented. The results from cyclopropanation show that SF5 groups dramatically improved the yields and stereoselectivity compared to the CF3.
- This article is part of the themed collection: Most popular 2021 main group, inorganic and organometallic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...