Inhibition of (dppf)nickel-catalysed Suzuki–Miyaura cross-coupling reactions by α-halo-N-heterocycles†
Abstract
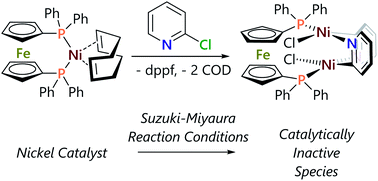
A nickel/dppf catalyst system was found to successfully achieve the Suzuki–Miyaura cross-coupling reactions of 3- and 4-chloropyridine and of 6-chloroquinoline but not of 2-chloropyridine or of other α-halo-N-heterocycles. Further investigations revealed that chloropyridines undergo rapid oxidative addition to [Ni(COD)(dppf)] but that α-halo-N-heterocycles lead to the formation of stable dimeric nickel species that are catalytically inactive in Suzuki–Miyaura cross-coupling reactions. However, the corresponding Kumada–Tamao–Corriu reactions all proceed readily, which is attributed to more rapid transmetalation of Grignard reagents.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...