Structure–function relationships in aryl diazirines reveal optimal design features to maximize C–H insertion†
Abstract
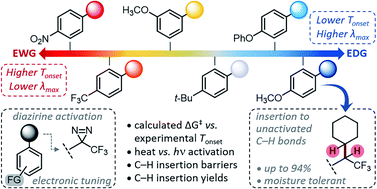
Diazirine reagents allow for the ready generation of carbenes upon photochemical, thermal, or electrical stimulation. Because carbenes formed in this way can undergo rapid insertion into any nearby C–H, O–H or N–H bond, molecules that encode diazirine functions have emerged as privileged tools in applications ranging from biological target identification and proteomics through to polymer crosslinking and adhesion. Here we use a combination of experimental and computational methods to complete the first comprehensive survey of diazirine structure–function relationships, with a particular focus on thermal activation methods. We reveal a striking ability to vary the activation energy and activation temperature of aryl diazirines through the rational manipulation of electronic properties. Significantly, we show that electron-rich diazirines have greatly enhanced efficacy toward C–H insertion, under both thermal and photochemical activation conditions. We expect these results to lead to significant improvements in diazirine-based chemical probes and polymer crosslinkers.


 Please wait while we load your content...
Please wait while we load your content...