Ion polarisation-assisted hydrogen-bonded ferroelectrics in liquid crystalline domains†
Abstract
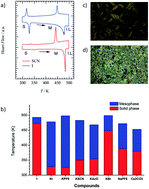
An alkylamide-substituted (−NHCOC10H21) hydrogen-bonded dibenzo[18]crown-6 derivative (1) was prepared to stabilise the ionic channel structure in a discotic hexagonal columnar (Colh) liquid crystal. The introduction of simple M+X− salts such as Na+PF6− and K+I− into the ionic channel of 1 enhanced the ionic conductivity of the Colh phase of the M+·(1)·X− salts, with the highest ionic conductivity reaching ∼10−6 S cm−1 for K+·(1)·I− and Na+·(1)·PF6− at 460 K, which was approximately 5 orders of magnitude higher than that of 1. The introduction of non-ferroelectric 1 into the ferroelectric N,N′,N′′-tri(tetradecyl)-1,3,5-benzenetricarboxamide (3BC) elicited a ferroelectric response from the mixed Colh phase of (3BC)x(1)1−x with x = 0.9 and 0.8. The further doping of M+X− into the ferroelectric Colh phase of (3BC)0.9(1)0.1 enhanced the ferroelectric polarisation assisted by ion displacement in the half-filled ionic channel for the vacant dibenzo[18]crown-6 of (3BC)0.9[(M+)0.5·(1)·(X−)0.5]0.1.


 Please wait while we load your content...
Please wait while we load your content...