Biphasic electrochemical peptide synthesis†
Abstract
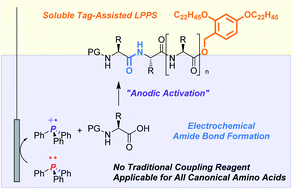
The large amount of waste derived from coupling reagents is a serious drawback of peptide synthesis from a green chemistry viewpoint. To overcome this issue, we report an electrochemical peptide synthesis in a biphasic system. Anodic oxidation of triphenylphosphine (Ph3P) generates a phosphine radical cation, which serves as the coupling reagent to activate carboxylic acids, and produces triphenylphosphine oxide (Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) as a stoichiometric byproduct. In combination with a soluble tag-assisted liquid-phase peptide synthesis, the selective recovery of desired peptides and Ph3P
O) as a stoichiometric byproduct. In combination with a soluble tag-assisted liquid-phase peptide synthesis, the selective recovery of desired peptides and Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was achieved. Given that methods to reduce Ph3P
O was achieved. Given that methods to reduce Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to Ph3P have been reported, Ph3P
O to Ph3P have been reported, Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O could be a recyclable byproduct unlike byproducts from typical coupling reagents. Moreover, a commercial peptide active pharmaceutical ingredient (API), leuprorelin, was successfully synthesized without the use of traditional coupling reagents.
O could be a recyclable byproduct unlike byproducts from typical coupling reagents. Moreover, a commercial peptide active pharmaceutical ingredient (API), leuprorelin, was successfully synthesized without the use of traditional coupling reagents.


 Please wait while we load your content...
Please wait while we load your content...