Combining flavin photocatalysis with parallel synthesis: a general platform to optimize peptides with non-proteinogenic amino acids†
Abstract
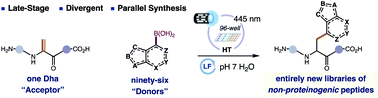
Most peptide drugs contain non-proteinogenic amino acids (NPAAs), born out through extensive structure–activity relationship (SAR) studies using solid-phase peptide synthesis (SPPS). Synthetically laborious and expensive to manufacture, NPAAs also can have poor coupling efficiencies allowing only a small fraction to be sampled by conventional SPPS. To gain general access to NPAA-containing peptides, we developed a first-generation platform that merges contemporary flavin photocatalysis with parallel synthesis to simultaneously make, purify, quantify, and even test up to 96 single-NPAA peptide variants via the unique combination of boronic acids and a dehydroalanine residue in a peptide. We showcase the power of our newly minted platform to introduce NPAAs of diverse chemotypes-aliphatic, aromatic, heteroaromatic-directly into peptides, including 15 entirely new residues, and to evolve a simple proteinogenic peptide into an unnatural inhibitor of thrombin by non-classical peptide SAR.


 Please wait while we load your content...
Please wait while we load your content...