Quinol-containing ligands enable high superoxide dismutase activity by modulating coordination number, charge, oxidation states and stability of manganese complexes throughout redox cycling†
Abstract
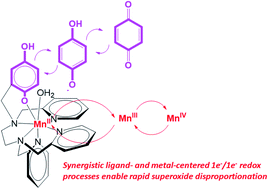
Reactivity assays previously suggested that two quinol-containing MRI contrast agent sensors for H2O2, [Mn(H2qp1)(MeCN)]2+ and [Mn(H4qp2)Br2], could also catalytically degrade superoxide. Subsequently, [Zn(H2qp1)(OTf)]+ was found to use the redox activity of the H2qp1 ligand to catalyze the conversion of O2˙− to O2 and H2O2, raising the possibility that the organic ligand, rather than the metal, could serve as the redox partner for O2˙− in the manganese chemistry. Here, we use stopped-flow kinetics and cryospray-ionization mass spectrometry (CSI-MS) analysis of the direct reactions between the manganese-containing contrast agents and O2˙− to confirm the activity and elucidate the catalytic mechanism. The obtained data are consistent with the operation of multiple parallel catalytic cycles, with both the quinol groups and manganese cycling through different oxidation states during the reactions with superoxide. The choice of ligand impacts the overall charges of the intermediates and allows us to visualize complementary sets of intermediates within the catalytic cycles using CSI-MS. With the diquinolic H4qp2, we detect Mn(III)-superoxo intermediates with both reduced and oxidized forms of the ligand, a Mn(III)-hydroperoxo compound, and what is formally a Mn(IV)-oxo species with the monoquinolate/mono-para-quinone form of H4qp2. With the monoquinolic H2qp1, we observe a Mn(II)-superoxo ↔ Mn(III)-peroxo intermediate with the oxidized para-quinone form of the ligand. The observation of these species suggests inner-sphere mechanisms for O2˙− oxidation and reduction that include both the ligand and manganese as redox partners. The higher positive charges of the complexes with the reduced and oxidized forms of H2qp1 compared to those with related forms of H4qp2 result in higher catalytic activity (kcat ∼ 108 M−1 s−1 at pH 7.4) that rivals those of the most active superoxide dismutase (SOD) mimics. The manganese complex with H2qp1 is markedly more stable in water than other highly active non-porphyrin-based and even some Mn(II) porphyrin-based SOD mimics.


 Please wait while we load your content...
Please wait while we load your content...