Desymmetrization of pibrentasvir for efficient prodrug synthesis†
Abstract
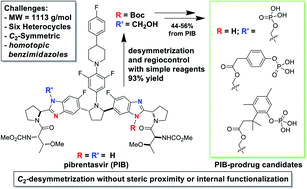
A novel and practical desymmetrization tactic is described to access a new class of pibrentasvir prodrugs. The homotopic benzimidazoles of pibrentasvir (PIB) are differentiated via a one-pot di-Boc/mono-de-Boc selective N-Boc protection and formaldehyde adduct formation sequence, both enabled by crystallization-induced selectivity. The first step represents the only known application of the Horeau principle of statistical amplification for C2-symmetric polyheterocycle regioselective functionalization. The resulting versatile intermediate is employed in the high-yielding preparation of several pibrentasvir prodrug candidates.


 Please wait while we load your content...
Please wait while we load your content...