Modulation of amyloid-β aggregation by metal complexes with a dual binding mode and their delivery across the blood–brain barrier using focused ultrasound†
Abstract
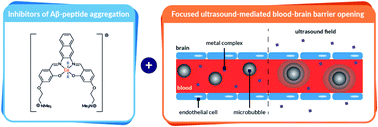
One of the key hallmarks of Alzheimer's disease is the aggregation of the amyloid-β peptide to form fibrils. Consequently, there has been great interest in studying molecules that can disrupt amyloid-β aggregation. While a handful of molecules have been shown to inhibit amyloid-β aggregation in vitro, there remains a lack of in vivo data reported due to their inability to cross the blood–brain barrier. Here, we investigate a series of new metal complexes for their ability to inhibit amyloid-β aggregation in vitro. We demonstrate that octahedral cobalt complexes with polyaromatic ligands have high inhibitory activity thanks to their dual binding mode involving π–π stacking and metal coordination to amyloid-β (confirmed via a range of spectroscopic and biophysical techniques). In addition to their high activity, these complexes are not cytotoxic to human neuroblastoma cells. Finally, we report for the first time that these metal complexes can be safely delivered across the blood–brain barrier to specific locations in the brains of mice using focused ultrasound.
- This article is part of the themed collection: Most popular 2021 main group, inorganic and organometallic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...