Direct synthesis of pentasubstituted pyrroles and hexasubstituted pyrrolines from propargyl sulfonylamides and allenamides†
Abstract
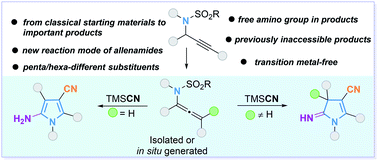
Multisubstituted pyrroles are important fragments that appear in many bioactive small molecule scaffolds. Efficient synthesis of multisubstituted pyrroles with different substituents from easily accessible starting materials is challenging. Herein, we describe a metal-free method for the preparation of pentasubstituted pyrroles and hexasubstituted pyrrolines with different substituents and a free amino group by a base-promoted cascade addition–cyclization of propargylamides or allenamides with trimethylsilyl cyanide. This method would complement previous methods and support expansion of the toolbox for the synthesis of valuable, but previously inaccessible, highly substituted pyrroles and pyrrolines. Mechanistic studies to elucidate the reaction pathway have been conducted.


 Please wait while we load your content...
Please wait while we load your content...