Regioselective difunctionalization of pyridines via 3,4-pyridynes†
Abstract
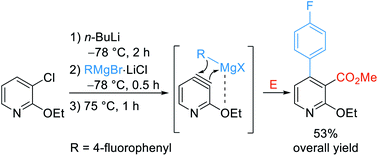
A new regioselective 3,4-difunctionalization of 3-chloropyridines via 3,4-pyridyne intermediates is reported. Regioselective lithiation of 3-chloro-2-ethoxypyridine and a related 2-thio-derivative followed by treatment with aryl- and alkylmagnesium halides as well as magnesium thiolates at −78 °C produced 3,4-pyridynes during heating to 75 °C. Regioselective addition of the Grignard moiety in position 4 followed by an electrophilic quench in position 3 led to various 2,3,4-trisubstituted pyridines. This method was adapted into a continuous flow set-up. As an application, we have prepared a key intermediate for (±)-paroxetine.


 Please wait while we load your content...
Please wait while we load your content...