Effect of the abolition of intersubunit salt bridges on allosteric protein structural dynamics†
Abstract
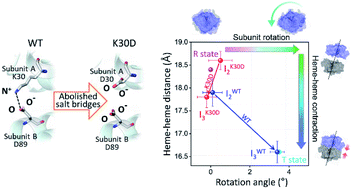
A salt bridge, one of the representative structural factors established by non-covalent interactions, plays a crucial role in stabilizing the structure and regulating the protein function, but its role in dynamic processes has been elusive. Here, to scrutinize the structural and functional roles of the salt bridge in the process of performing the protein function, we investigated the effects of salt bridges on the allosteric structural transition of homodimeric hemoglobin (HbI) by applying time-resolved X-ray solution scattering (TRXSS) to the K30D mutant, in which the interfacial salt bridges of the wild type (WT) are abolished. The TRXSS data of K30D are consistent with the kinetic model that requires one monomer intermediate in addition to three structurally distinct dimer intermediates (I1, I2, and I3) observed in WT and other mutants. The kinetic and structural analyses show that K30D has an accelerated biphasic transition from I2 to I3 by more than nine times compared to WT and lacks significant structural changes in the transition from R-like I2 to T-like I3 observed in WT, unveiling that the loss of the salt bridges interrupts the R–T allosteric transition of HbI. Besides, the correlation between the bimolecular CO recombination rates in K30D, WT, and other mutants reveals that the bimolecular CO recombination is abnormally decelerated in K30D, indicating that the salt bridges also affect the cooperative ligand binding in HbI. These comparisons of the structural dynamics and kinetics of K30D and WT show that the interfacial salt bridges not only assist the physical connection of two subunits but also play a critical role in the global structural signal transduction of one subunit to the other subunit via a series of well-organized structural transitions.


 Please wait while we load your content...
Please wait while we load your content...