Cooperative copper-squaramide catalysis for the enantioselective N–H insertion reaction with sulfoxonium ylides†
Abstract
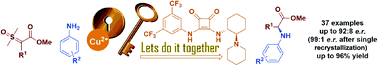
The first examples of a highly efficient and enantioselective carbene-mediated insertion reaction, from a sulfur ylide, are described. By way of a catalytic asymmetric insertion reaction into N–H bonds from carbonyl sulfoxonium ylides and anilines, using a copper-bifunctional squaramide cooperative catalysis approach, thirty-seven α-arylglycine esters were synthesized in enantiomeric ratios up to 92 : 8 (99 : 1 after a single recrystallization) and reaction yields ranging between 49–96%. Furthermore, the protocol benefits from quick reaction times and is conducted in a straightforward manner.


 Please wait while we load your content...
Please wait while we load your content...