A bifunctional iminophosphorane squaramide catalyzed enantioselective synthesis of hydroquinazolines via intramolecular aza-Michael reaction to α,β-unsaturated esters†
Abstract
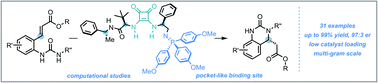
An efficient synthesis of enantioenriched hydroquinazoline cores via a novel bifunctional iminophosphorane squaramide catalyzed intramolecular aza-Michael reaction of urea-linked α,β-unsaturated esters is described. The methodology exhibits a high degree of functional group tolerance around the forming hydroquinazoline aryl core and wide structural variance on the nucleophilic N atom of the urea moiety. Excellent yields (up to 99%) and high enantioselectivities (up to 97 : 3 er) using both aromatic and less acidic aliphatic ureas were realized. The potential industrial applicability of the transformation was demonstrated in a 20 mmol scale-up experiment using an adjusted catalyst loading of 2 mol%. The origin of enantioselectivity and reactivity enhancement provided by the squaramide motif has been uncovered computationally using density functional theory (DFT) calculations, combined with the activation strain model (ASM) and energy decomposition analysis (EDA).
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...