Submonomer synthesis of peptoids containing trans-inducing N-imino- and N-alkylamino-glycines†
Abstract
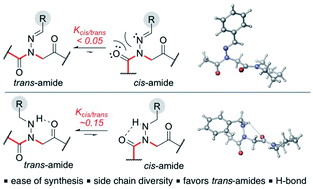
The use of hydrazones as a new type of submonomer in peptoid synthesis is described, giving access to peptoid monomers that are structure-inducing. A wide range of hydrazones were found to readily react with α-bromoamides in routine solid phase peptoid submonomer synthesis. Conditions to promote a one-pot cleavage of the peptoid from the resin and reduction to the corresponding N-alkylamino side chains were also identified, and both the N-imino- and N-alkylamino glycine residues were found to favor the trans-amide bond geometry by NMR, X-ray crystallography, and computational analyses.


 Please wait while we load your content...
Please wait while we load your content...