Exploiting host–guest chemistry to manipulate magnetic interactions in metallosupramolecular M4L6 tetrahedral cages†
Abstract
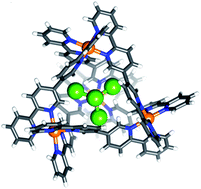
Reaction of Ni(OTf)2 with the bisbidentate quaterpyridine ligand L results in the self-assembly of a tetrahedral, paramagnetic cage [NiII4L6]8+. By selectively exchanging the bound triflate from [OTf⊂NiII4L6](OTf)7 (1), we have been able to prepare a series of host–guest complexes that feature an encapsulated paramagnetic tetrahalometallate ion inside this paramagnetic host giving [MIIX4⊂NiII4L6](OTf)6, where MIIX42− = MnCl42− (2), CoCl42− (5), CoBr42− (6), NiCl42− (7), and CuBr42− (8) or [MIIIX4⊂NiII4L6](OTf)7, where MIIIX4− = FeCl4− (3) and FeBr4− (4). Triflate-to-tetrahalometallate exchange occurs in solution and can also be accomplished through single-crystal-to-single-crystal transformations. Host–guest complexes 1–8 all crystallise as homochiral racemates in monoclinic space groups, wherein the four {NiN6} vertexes within a single Ni4L6 unit possess the same Δ or Λ stereochemistry. Magnetic susceptibility and magnetisation data show that the magnetic exchange between metal ions in the host [NiII4] complex, and between the host and the MX4n− guest, are of comparable magnitude and antiferromagnetic in nature. Theoretically derived values for the magnetic exchange are in close agreement with experiment, revealing that large spin densities on the electronegative X-atoms of particular MX4n− guest molecules lead to stronger host–guest magnetic exchange interactions.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...