Reversible metathesis of ammonia in an acyclic germylene–Ni0 complex†
Abstract
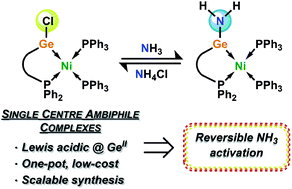
Carbenes, a class of low-valent group 14 ligand, have shifted the paradigm in our understanding of the effects of supporting ligands in transition-metal reactivity and catalysis. We now seek to move towards utilizing the heavier group 14 elements in effective ligand systems, which can potentially surpass carbon in their ability to operate via ‘non-innocent’ bond activation processes. Herein we describe our initial results towards the development of scalable acyclic chelating germylene ligands (viz.1a/b), and their utilization in the stabilization of Ni0 complexes (viz.4a/b), which can readily and reversibly undergo metathesis with ammonia with no net change of oxidation state at the GeII and Ni0 centres, through ammonia bonding at the germylene ligand as opposed to the Ni0 centre. The DFT-derived metathesis mechanism, which surprisingly demonstrates the need for three molecules of ammonia to achieve N–H bond activation, supports reversible ammonia binding at GeII, as well as the observed reversibility in the overall reaction.
- This article is part of the themed collection: Most popular 2021 main group, inorganic and organometallic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...