Boosting PLA melt strength by controlling the chirality of co-monomer incorporation†
Abstract
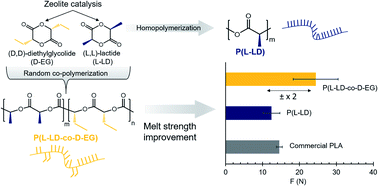
Bio-based and degradable polymers such as poly(lactic acid) (PLA) have become prominent. In spite of encouraging features, PLA has a low melt strength and melt elasticity, resulting in processing and application limitations that diminish its substitution potential vis-a-vis classic plastics. Here, we demonstrate a large increase in zero shear viscosity, melt elasticity, elongational viscosity and melt strength by random co-polymerization of lactide with small amounts, viz. 0.4–10 mol%, of diethylglycolide of opposite chiral nature. These enantiomerically pure monomers can be synthesized using one-step zeolite catalysis. Screening of the ester linkages in the final PLA chains by the ethyl side groups is suggested to create an expanding effect on the polymer coils in molten state by weakening of chain–chain interactions. This effect is suspected to increase the radius of gyration, enabling more chain entanglements and consequently increasing the melt strength. A stronger melt could enable access to more cost-competitive and sustainable PLA-based biomaterials with a broader application window. Amongst others, blow molding of bottles, film blowing, fiber spinning and foaming could be facilitated by PLA materials exhibiting a higher melt strength.


 Please wait while we load your content...
Please wait while we load your content...