Oxanorbornenes: promising new single addition monomers for the metathesis polymerization†
Abstract
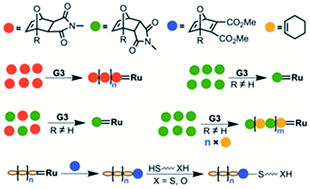
Higher ring-opening metathesis propagation rates of exo-norbornene derivatives over endo derivatives are well established in the literature. Here, we report for the first time that endo-isomers of oxanorbornene derivatives show higher reactivity towards ring-opening metathesis with Grubbs' 3rd generation catalyst (G3) than the corresponding exo-isomers. A very high selectivity for the reaction of G3 with endo over the exo-isomers could be shown. Furthermore, single molecular addition of the endo-isomers with G3 was observed. On the other hand, pure exo-monomers could successfully be homopolymerized. Mixtures of exo- and endo- monomers, however, prevented the homopolymerization of the exo-monomer. Such mixtures could successfully be copolymerized with cycloalkenes, resulting in alternating copolymers. An oxanorbornadiene derivative could be shown to undergo single addition reactions, exploited in the preparation of mono-end functional ROMP polymers. These could be selectively derivatized via endgroup selective thiol-ene click reactions. A thiol and alcohol end functional ROMP polymer was synthesized, and the efficient end functionalization was confirmed by 1H NMR spectroscopy and MALDI-ToF spectrometry.


 Please wait while we load your content...
Please wait while we load your content...