Abstract
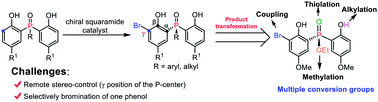
A novel and efficient desymmetrizing asymmetric ortho-selective mono-bromination of bisphenol phosphine oxides under chiral squaramide catalysis was reported. Using this asymmetric ortho-bromination strategy, a wide range of chiral bisphenol phosphine oxides and bisphenol phosphinates were obtained with good to excellent yields (up to 92%) and enantioselectivities (up to 98.5 : 1.5 e.r.). The reaction could be scaled up, and the synthetic utility of the desired P-stereogenic compounds was proved by transformations and application in an asymmetric reaction.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...