Stalling chromophore synthesis of the fluorescent protein Venus reveals the molecular basis of the final oxidation step†
Abstract
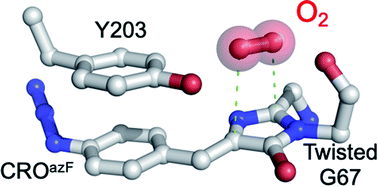
Fluorescent proteins (FPs) have revolutionised the life sciences, but the mechanism of chromophore maturation is still not fully understood. Here we show that incorporation of a photo-responsive non-canonical amino acid within the chromophore stalls maturation of Venus, a yellow FP, at an intermediate stage; a crystal structure indicates the presence of O2 located above a dehydrated enolate form of the imidazolone ring, close to the strictly conserved Gly67 that occupies a twisted conformation. His148 adopts an “open” conformation so forming a channel that allows O2 access to the immature chromophore. Absorbance spectroscopy supported by QM/MM simulations suggests that the first oxidation step involves formation of a hydroperoxyl intermediate in conjunction with dehydrogenation of the methylene bridge. A fully conjugated mature chromophore is formed through release of H2O2, both in vitro and in vivo. The possibility of interrupting and photochemically restarting chromophore maturation and the mechanistic insights open up new approaches for engineering optically controlled fluorescent proteins.


 Please wait while we load your content...
Please wait while we load your content...