Nanoscale electrochemistry in a copper/aqueous/oil three-phase system: surface structure–activity-corrosion potential relationships†
Abstract
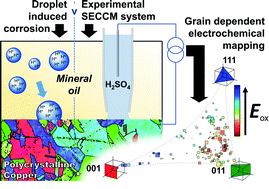
Practically important metal electrodes are usually polycrystalline, comprising surface grains of many different crystallographic orientations, as well as grain boundaries. In this study, scanning electrochemical cell microscopy (SECCM) is applied in tandem with co-located electron backscattered diffraction (EBSD) to give a holistic view of the relationship between the surface structure and the electrochemical activity and corrosion susceptibility of polycrystalline Cu. An unusual aqueous nanodroplet/oil (dodecane)/metal three-phase configuration is employed, which opens up new prospects for fundamental studies of multiphase electrochemical systems, and mimics the environment of corrosion in certain industrial and automotive applications. In this configuration, the nanodroplet formed at the end of the SECCM probe (nanopipette) is surrounded by dodecane, which acts as a reservoir for oil-soluble species (e.g., O2) and can give rise to enhanced flux(es) across the immiscible liquid–liquid interface, as shown by finite element method (FEM) simulations. This unique three-phase configuration is used to fingerprint nanoscale corrosion in a nanodroplet cell, and to analyse the interrelationship between the Cu oxidation, Cu2+ deposition and oxygen reduction reaction (ORR) processes, together with nanoscale open circuit (corrosion) potential, in a grain-by-grain manner. Complex patterns of surface reactivity highlight the important role of grains of high-index orientation and microscopic surface defects (e.g., microscratches) in modulating the corrosion-properties of polycrystalline Cu. This work provides a roadmap for in-depth surface structure–function studies in (electro)materials science and highlights how small variations in surface structure (e.g., crystallographic orientation) can give rise to large differences in nanoscale reactivity.


 Please wait while we load your content...
Please wait while we load your content...