Activation of ammonia and hydrazine by electron rich Fe(ii) complexes supported by a dianionic pentadentate ligand platform through a common terminal Fe(iii) amido intermediate†‡
Abstract
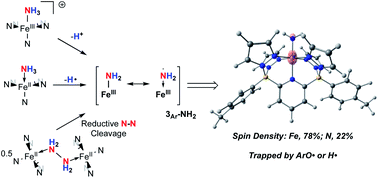
We report the use of electron rich iron complexes supported by a dianionic diborate pentadentate ligand system, B2Pz4Py, for the coordination and activation of ammonia (NH3) and hydrazine (NH2NH2). For ammonia, coordination to neutral (B2Pz4Py)Fe(II) or cationic [(B2Pz4Py)Fe(III)]+ platforms leads to well characterized ammine complexes from which hydrogen atoms or protons can be removed to generate, fleetingly, a proposed (B2Pz4Py)Fe(III)–NH2 complex (3Ar-NH2). DFT computations suggest a high degree of spin density on the amido ligand, giving it significant aminyl radical character. It rapidly traps the H atom abstracting agent 2,4,6-tri-tert-butylphenoxy radical (ArO˙) to form a C–N bond in a fully characterized product (2Ar), or scavenges hydrogen atoms to return to the ammonia complex (B2Pz4Py)Fe(II)–NH3 (1Ar-NH3). Interestingly, when (B2Pz4Py)Fe(II) is reacted with NH2NH2, a hydrazine bridged dimer, (B2Pz4Py)Fe(II)–NH2NH2–Fe(II)(B2Pz4Py) ((1Ar)2-NH2NH2), is observed at −78 °C and converts to a fully characterized bridging diazene complex, 4Ar, along with ammonia adduct 1Ar-NH3 as it is allowed to warm to room temperature. Experimental and computational evidence is presented to suggest that (B2Pz4Py)Fe(II) induces reductive cleavage of the N–N bond in hydrazine to produce the Fe(III)–NH2 complex 3Ar-NH2, which abstracts H˙ atoms from (1Ar)2-NH2NH2 to generate the observed products. All of these transformations are relevant to proposed steps in the ammonia oxidation reaction, an important process for the use of nitrogen-based fuels enabled by abundant first row transition metals.


 Please wait while we load your content...
Please wait while we load your content...