Traceless parallel peptide purification by a first-in-class reductively cleavable linker system featuring a safety-release†
Abstract
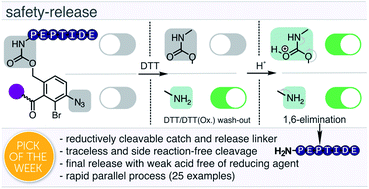
Hundreds of peptides can be synthesized by automated parallel synthesizers in a single run. In contrast, the most widely used peptide purification method – high-pressure liquid chromatography (HPLC) – only allows one-by-one processing of each sample. The chromatographic purification of many peptides, therefore, remains a time-consuming and costly effort. Catch-and-release methods can be processed in parallel and potentially provide a remedy. However, no such system has yet provided a true alternative to HPLC. Herein we present the development of a side-reaction free, reductively cleavable linker. The linker is added to the target peptide as the last building block during peptide synthesis. After acidic cleavage from synthetic resin, the linker-tagged full-length peptide is caught onto an aldehyde-modified solid support by rapid oxime ligation, allowing removal of all impurities lacking the linker by washing. Reducing the aryl azide to an aniline sensitizes the linker for cleavage. However, scission does not occur at non-acidic pH enabling wash out of reducing agent. Final acidic treatment safely liberates the peptide by an acid-catalysed 1,6-elimination. We showcase this first-in-class reductively cleavable linker system in the parallel purification of a personalized neoantigen cocktail, containing 20 peptides for cancer immunotherapy within six hours.
- This article is part of the themed collections: 2021 ChemSci Pick of the Week Collection and 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...