Total synthesis of biselide A†
Abstract
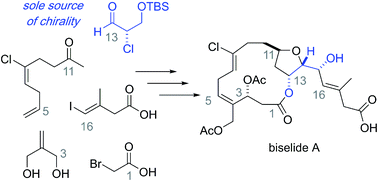
A total synthesis of the marine macrolide biselide A is described that relies on an enantiomerically enriched α-chloroaldehyde as the sole chiral building block. Several strategies to construct the macrocycle are presented including a macrocyclic Reformatsky reaction that ultimately provides access to the natural product in a longest linear sequence of 18 steps. Biological testing of synthetic biselide A suggests this macrolide disrupts cell division through a mechanism related to the regulation of microtubule cytoskeleton organization. Overall, this concise synthesis and insight gained into the mechanism of action should inspire medicinal chemistry efforts directed at structurally related anticancer marine macrolides.


 Please wait while we load your content...
Please wait while we load your content...