Hydantoin-bridged medium ring scaffolds by migratory insertion of urea-tethered nitrile anions into aromatic C–N bonds†
Abstract
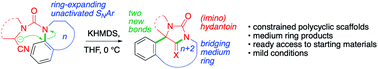
Bicyclic or tricyclic nitrogen-containing heterocyclic scaffolds were constructed rapidly by intramolecular nucleophilic aromatic substitution of metallated nitriles tethered by a urea linkage to a series of electronically unactivated heterocyclic precursors. The substitution reaction constitutes a ring expansion, enabled by the conformationally constrained tether between the nitrile and the heterocycle. Attack of the metallated urea leaving group on the nitrile generates a hydantoin that bridges the polycyclic products. X-ray crystallography reveals ring-dependant strain within the hydantoin.


 Please wait while we load your content...
Please wait while we load your content...