Catalytic enantioselective synthesis of macrodiolides and their application in chiral recognition†
Abstract
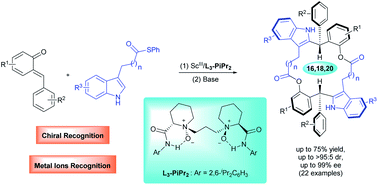
New types of C2-symmetric chiral macrodiolides are readily obtained via chiral N,N′-dioxide-scandium(III) complex-promoted asymmetric tandem Friedel–Crafts alkylation/intermolecular macrolactonization of ortho-quinone methides with C3-substituted indoles. This protocol provides an array of enantioenriched macrodiolides with 16, 18 or 20-membered rings in moderate to good yields with high diastereoselectivities and excellent enantioselectivities through adjusting the length of the tether at the C3 position of indoles. Density functional theory calculations indicate that the formation of macrocycles is more favorable than that of 9-membered-ring lactones in terms of kinetics and thermodynamics. The potential utility of these intriguing chiral macrodiolide molecules is demonstrated in the enantiomeric recognition of aminols and chemical recognition of metal ions.


 Please wait while we load your content...
Please wait while we load your content...