Tandem diaza-Cope rearrangement polymerization: turning intramolecular reaction into powerful polymerization to give enantiopure materials for Zn2+ sensors†
Abstract
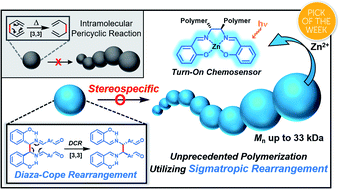
[3,3]-Sigmatropic rearrangement is a powerful reaction to form C–C bonds stereospecifically; however, owing to intrinsic simultaneous bond formation and breakage, this versatile method has not been utilized in polymerization. Herein, we report a new tandem diaza-Cope rearrangement polymerization (DCRP) that can synthesize polymers with defect-free C–C bond formation from easy and efficient imine formation. A mechanistic investigation by in situ1H NMR experiments suggests that this polymerization proceeds by a rapid DCR process, forming an enantiospecific C–C bond that occurs almost simultaneously with imine formation. This polymerization produces not only highly stable polymers against hydrolysis due to resonance-assisted hydrogen bonds (RAHBs) but also chiral polymers containing enantiopure salen moieties, which lead to high-performance Zn2+-selective turn-on chemosensors with up to 73-fold amplification. We also found that their optical activities and sensing performances are heavily dependent on the reaction temperature, which significantly affects the stereoselectivity of DCR.
- This article is part of the themed collections: 2020 ChemSci Pick of the Week Collection and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...