Real-time imaging of nanoscale electrochemical Ni etching under thermal conditions†
Abstract
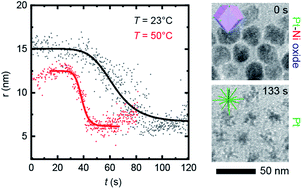
The ability to vary the temperature of an electrochemical cell provides opportunities to control reaction rates and pathways and to drive processes that are inaccessible at ambient temperature. Here, we explore the effect of temperature on electrochemical etching of Ni–Pt bimetallic nanoparticles. To observe the process at nanoscale resolution we use liquid cell transmission electron microscopy with a modified liquid cell that enables simultaneous heating and biasing. By controlling the cell temperature, we demonstrate that the reaction rate and dissolution potential of the electrochemical Ni etching process can be changed. The in situ measurements suggest that the destabilization of the native nickel oxide layer is the slow step prior to subsequent fast Ni removal in the electrochemical Ni dissolution process. These experiments highlight the importance of in situ structural characterization under electrochemical and thermal conditions as a strategy to provide deeper insights into nanomaterial transformations as a function of temperature and potential.


 Please wait while we load your content...
Please wait while we load your content...