Mild olefin formation via bio-inspired vitamin B12 photocatalysis†
Abstract
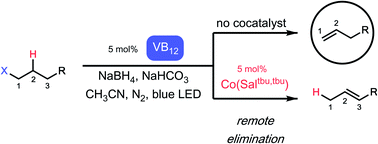
Dehydrohalogenation, or elimination of hydrogen-halide equivalents, remains one of the simplest methods for the installation of the biologically-important olefin functionality. However, this transformation often requires harsh, strongly-basic conditions, rare noble metals, or both, limiting its applicability in the synthesis of complex molecules. Nature has pursued a complementary approach in the novel vitamin B12-dependent photoreceptor CarH, where photolysis of a cobalt–carbon bond leads to selective olefin formation under mild, physiologically-relevant conditions. Herein we report a light-driven B12-based catalytic system that leverages this reactivity to convert alkyl electrophiles to olefins under incredibly mild conditions using only earth abundant elements. Further, this process exhibits a high level of regioselectivity, producing terminal olefins in moderate to excellent yield and exceptional selectivity. Finally, we are able to access a hitherto-unknown transformation, remote elimination, using two cobalt catalysts in tandem to produce subterminal olefins with excellent regioselectivity. Together, we show vitamin B12 to be a powerful platform for developing mild olefin-forming reactions.


 Please wait while we load your content...
Please wait while we load your content...