Single point activation of pyridines enables reductive hydroxymethylation†
Abstract
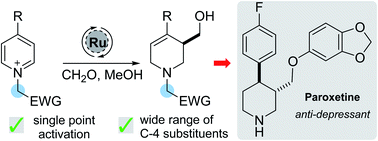
The single point activation of pyridines, using an electron-deficient benzyl group, facilitates the ruthenium-catalysed dearomative functionalisation of a range of electronically diverse pyridine derivatives. This transformation delivers hydroxymethylated piperidines in good yields, allowing rapid access to medicinally relevant small heterocycles. A noteworthy feature of this work is that paraformaldehyde acts as both a hydride donor and an electrophile in the reaction, enabling the use of cheap and readily available feedstock chemicals. Removal of the activating group can be achieved readily, furnishing the free NH compound in only 2 steps. The synthetic utility of the method was illustrated with a synthesis of (±)-Paroxetine.


 Please wait while we load your content...
Please wait while we load your content...