Introducing affinity and selectivity into galectin-targeting nanoparticles with fluorinated glycan ligands†
Abstract
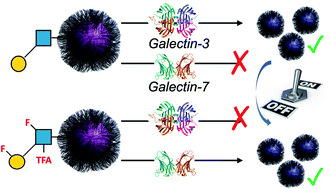
Galectins are potential biomarkers and therapeutic targets. However, galectins display broad affinity towards β-galactosides meaning glycan-based (nano)biosensors lack the required selectivity and affinity. Using a polymer-stabilized nanoparticle biosensing platform, we herein demonstrate that the specificity of immobilised lacto-N-biose towards galectins can be ‘turned on/off’ by using site-specific glycan fluorination and in some cases reversal of specificity can be achieved. The panel of fluoro-glycans were obtained by a chemoenzymatic approach, exploiting BiGalK and BiGalHexNAcP enzymes from Bifidobacterium infantis which are shown to tolerate fluorinated glycans, introducing structural diversity which would be very laborious by chemical methods alone. These results demonstrate that integrating non-natural, fluorinated glycans into nanomaterials can encode unprecedented selectivity with potential applications in biosensing.
- This article is part of the themed collections: Celebrating our 2021 Prizewinners and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...