Does liquid–liquid phase separation drive peptide folding?†
Abstract
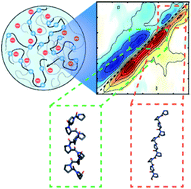
Proline–arginine (PR) dipeptide repeats have been shown to undergo liquid–liquid phase separation and are an example of a growing number of intrinsically disordered proteins that can assemble into membraneless organelles. These structures have been posited as nucleation sites for pathogenic protein aggregation. As such, a better understanding of the effects that the increased local concentration and volumetric crowding within droplets have on peptide secondary structure is necessary. Herein we use Fourier transform infrared (FTIR) and two-dimensional infrared (2DIR) spectroscopy to show that formation of droplets by PR20 accompanies changes in the amide-I spectra consistent with folding into poly-proline helical structures.


 Please wait while we load your content...
Please wait while we load your content...