Single-molecule fluorescence detection of a tricyclic nucleoside analogue†
Abstract
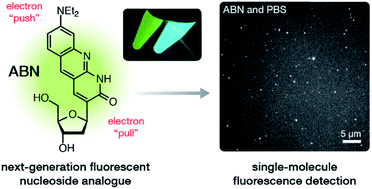
Fluorescent nucleobase surrogates capable of Watson–Crick hydrogen bonding are essential probes of nucleic acid structure and dynamics, but their limited brightness and short absorption and emission wavelengths have rendered them unsuitable for single-molecule detection. Aiming to improve on these properties, we designed a new tricyclic pyrimidine nucleoside analogue with a push–pull conjugated system and synthesized it in seven sequential steps. The resulting C-linked 8-(diethylamino)benzo[b][1,8]naphthyridin-2(1H)-one nucleoside, which we name ABN, exhibits ε442 = 20 000 M−1 cm−1 and Φem,540 = 0.39 in water, increasing to Φem = 0.50–0.53 when base paired with adenine in duplex DNA oligonucleotides. Single-molecule fluorescence measurements of ABN using both one-photon and two-photon excitation demonstrate its excellent photostability and indicate that the nucleoside is present to > 95% in a bright state with count rates of at least 15 kHz per molecule. This new fluorescent nucleobase analogue, which, in duplex DNA, is the brightest and most red-shifted known, is the first to offer robust and accessible single-molecule fluorescence detection capabilities.
- This article is part of the themed collection: Most popular 2021 analytical chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...