Sequential removal and recovery of cadmium ions (Cd2+) using photocatalysis and reduction crystallization from the aqueous phase
Abstract
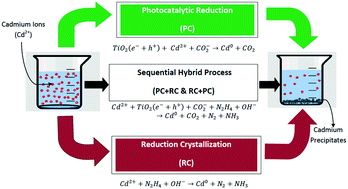
The toxic heavy metal cadmium (Cd) present in wastewater from chemical and industrial effluents shows persistence in aquatic media because of its non-degradability and is harmful to living organisms. A sequential method that uses photo-reduction in combination with reduction crystallization has been proposed for the effective removal and recovery of cadmium from the aqueous phase. Photocatalysis (PC) using titanium dioxide (TiO2) under optimized conditions (TiO2 2 g L−1, pH 7.2 and 35 W cm−2) removed 82.8% of cadmium ions (Cd2+) under UV light conditions, while the maximum removal of cadmium ions using reduction crystallization under optimized conditions (pH 10 and temp. 80 °C) was 88.2%. To attain maximum removal as well as recovery of cadmium (Cd), both processes were sequentially combined, removing 97.5% of cadmium in 120 min at 50 °C. The recovered catalysts (TiO2) and precipitates were characterized using different techniques such as scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). The XRD peaks and FT-IR analysis showed that the precipitates contained the cadmium element, whereas the XRD spectrum of recovered titanium dioxide (TiO2) indicated additional peaks at specific angles, showing cadmium deposition on the TiO2 surface. The rate of photocatalytic removal of cadmium ions (Cd2+) followed the Langmuir–Hinshelwood equation of the first order.


 Please wait while we load your content...
Please wait while we load your content...