Dynamics of phase transitions in Na2TiO3 and its possible utilization as a CO2 sorbent: a critical analysis
Abstract
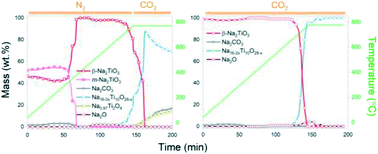
Na-Based materials are emerging as promising high-temperature CO2 sorbents. In this work, we provide a detailed study on the synthesis of Na2TiO3via a solid-state route using NaOH and TiO2 as starting reactants. The CO2 sorption properties of the synthesized Na2TiO3 were evaluated by thermogravimetric analysis. A subsequent comprehensive study on the complex reaction mechanism of Na2TiO3 at high temperatures under carbonation conditions was performed via real time in situ synchrotron X-ray diffraction analysis. In situ experiments performed under different conditions revealed the occurrence of thermally-driven phase transitions derived from the structural instability of the material at high temperatures. These reactions could be differentiated from carbonation processes, allowing the proposal of a reaction mechanism of the material as a CO2 sorbent. The obtained results can explain the abnormal dynamic thermogram displayed by Na2TiO3 in the presence of CO2 within a temperature range that is of interest for practical applications and serve as a basis for evaluating the feasibility of using this material in CO2 capture schemes.


 Please wait while we load your content...
Please wait while we load your content...