Non-thermal atmospheric pressure plasma as a powerful tool for the synthesis of rhenium-based nanostructures for the catalytic hydrogenation of 4-nitrophenol
Abstract
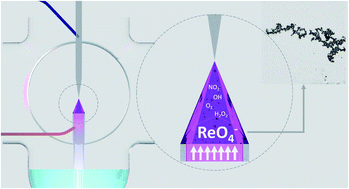
Here we have presented a new method for the synthesis of Re nanostructures with defined optical, structural, and catalytic properties. The Re-based nanoparticles (NPs) were obtained using a reaction-discharge system that is unique in its class, because of its working in the high-throughput mode. Within this application, direct current atmospheric pressure glow discharge (dc-APGD) was used as a non-thermal atmospheric pressure plasma (NTAP) source, which led to the reduction of Re(VII) ions and the formation of Re nanostructures through the plasma–liquid interactions. The Re-based NPs were synthesized in a flow-mode reaction-discharge system, where their precursor solution was a flowing liquid anode (FLA) or a flowing liquid cathode (FLC). The resultant NPs were analyzed using UV/Vis absorption spectrophotometry and transmission electron microscopy (TEM), which were supported by selected area X-ray diffraction (SAED) and the energy dispersive X-ray spectroscopy (EDX). Additionally, the mechanism for the reduction of Re(VII) ions was explained by the differences in the concentrations of the selected reactive nitrogen species (RNS) and reactive oxygen species (ROS) produced by dc-APGD. It was found that the application of dc-APGD, operating in a FLA configuration (FLA-dc-APGD), resulted in the formation of ReNPs with Re0, while the use of dc-APGD operating in a FLC configuration (FLC-dc-APGD) led to the formation of Re oxide NPs. In the latter case, a much greater oxidizing environment was likely provided, therefore the RNS and ROS contributed to the formation of Re oxide nanostructures. The ReNPs with Re0 were characterized by a size of 6.02 ± 3.01 nm, and the Re oxide NPs were characterized by a size of 4.97 ± 3.82 nm. Both types of nanostructures were then employed in the catalytic hydrogenation of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). Based on the results, both of the nanocatalysts effectively reduced 4-NP with an apparent rate constant (kapp) of 2.6 × 10−3 s−1. At the same time, the catalytic activity was linked with the average size distribution of the Re nanostructures, as opposed to their morphology.


 Please wait while we load your content...
Please wait while we load your content...