Photoelectron photofragment coincidence spectroscopy of carboxylates†
Abstract
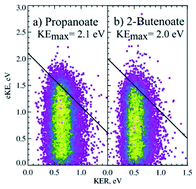
Photoelectron–photofragment coincidence (PPC) spectroscopy is a powerful technique for studying the decarboxylation dynamics of carboxyl radicals. Measurement of photoelectron and photofragment kinetic energies in coincidence provides a kinematically complete measure of the dissociative photodetachment (DPD) dynamics of carboxylate anions. PPC spectroscopy studies of methanoate, ethanoate, propanoate, 2-butenoate, benzoate, p-coumarate and the oxalate monoanion are reviewed. All of the systems studied undergo decarboxylation via a two-body DPD channel i.e. 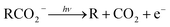 , driven by the thermodynamic stability of CO2. Additionally, decarboxylation is observed via a three-body ionic photodissociation channel for p-coumarate. In some cases photodetachment also results in a stable carboxyl radical (RCO2). The branching ratio for DPD, the threshold detachment energy and the peak of the kinetic energy release spectrum are compared for different carboxylates, as a probe of the character of the potential energy landscape in the Franck–Condon region.
, driven by the thermodynamic stability of CO2. Additionally, decarboxylation is observed via a three-body ionic photodissociation channel for p-coumarate. In some cases photodetachment also results in a stable carboxyl radical (RCO2). The branching ratio for DPD, the threshold detachment energy and the peak of the kinetic energy release spectrum are compared for different carboxylates, as a probe of the character of the potential energy landscape in the Franck–Condon region.
- This article is part of the themed collections: 2021 RSC Advances HOT Article Collection and 2021 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...