NS3/4A helicase inhibitory alkaloids from Aptenia cordifolia as HCV target†
Abstract
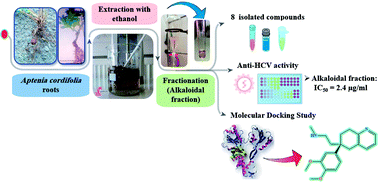
Chemical investigation of Aptenia cordifolia roots extract, using chromatographic and spectroscopic techniques, resulted in isolation and identification of eight known compounds. The basic ethyl acetate fraction (alkaloidal fraction) afforded O-methylsceletenone, epinine, 4-methoxy phenethylamine, and N-methyl tyramine while, the acidic ethyl acetate fraction (non-alkaloidal fraction) afforded only cis-N-coumaroyl tyramine. Moreover, the petroleum ether fraction afforded capric acid, tricosanol, and a mixture of β-sitosterol & stigma sterol. Upon screening of anti HCV activity of these three fractions, only the basic ethyl acetate fraction had high activity against HCV with an IC50 value equal to 2.4 μg mL−1 which provoked us to carry out structure based in silico virtual screening on the drug targets of HCV of isolated alkaloidal compounds as well as the previously dereplicated alkaloids through metabolomics from the antiviral active fraction. The tortuosamine compound exhibited the strongest binding to the active site of NS3/4A helicase with a binding affinity (−7.1 kcal mol−1) which is very close to the native ligand (−7.7 kcal mol−1).
- This article is part of the themed collection: 2021 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...