6-Halo-2-pyridone as an efficient organocatalyst for ester aminolysis†
Abstract
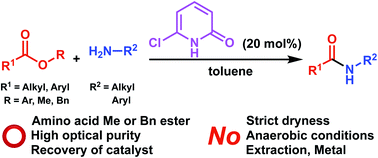
It was found that 6-halo-2-pyridones catalysed ester aminolysis in which not only reactive aryl esters but also relatively less reactive methyl and benzyl esters could be used as a substrate. The reaction could be performed without strictly dry and anaerobic conditions and the 6-chloro-2-pyridone catalyst could be recovered quantitatively after reaction. The method could be applied to dipeptide synthesis from methyl or benzyl esters of amino acids, where a high enantiomeric purity of the products was maintained. The mechanism involving dual activation of ester and amine substrates through hydrogen bonding between catalyst and substrates is proposed where 6-halo-2-pyridones act as a bifunctional Brønsted acid/base catalyst.


 Please wait while we load your content...
Please wait while we load your content...