Highly efficient sorption of molybdenum from tungstate solution with modified D301 resin
Abstract
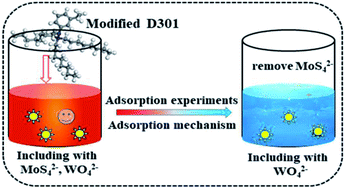
The separation of molybdenum (Mo) from tungstate solution is a bottleneck problem in tungsten (W) metallurgy, and it hinders the development of high-purity tungsten materials. In this research, a modified D301 resin was used to adsorb and separate molybdenum from tungstate solution. The maximum sorption capacity (Qe) of modified D301 for MoS42− was found to be 428 mg g−1 and the separation coefficient (β) was 108.9 when the contact time was 4 h and the reaction temperature was 25 °C and the pH value of the tungstate solution was 7.2. The sorption process conforms to Langmuir isotherm models and the quasi-second-order kinetic model. The sorption mechanism was also discussed, which was a single layered spontaneous sorption process. Theoretical calculations infer bonding behavior between the N atom on the resin and the S atom on the MoS42− molecule. The sorption energy is −7.67 eV, which indicated that the sorption process is stable chemical sorption. The desorption experiment showed that more than 90% molybdenum could be desorbed from the loaded resin when the concentration of sodium hydroxide solution was 5 w%. Finally, after three-stage sorption–desorption, almost all molybdenum in the solution was adsorbed, achieving better separation of tungsten and molybdenum.


 Please wait while we load your content...
Please wait while we load your content...