Synthesis of indazoles from 2-formylphenylboronic acids†
Abstract
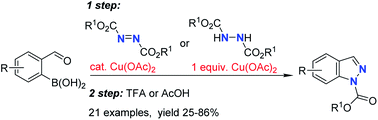
A method for the synthesis of indazoles was developed which involves a copper(II) acetate catalysed reaction of 2-formylboronic acids with diazadicaboxylates followed by acid or base induced ring closure. Hydrazine dicarboxylates were also shown as competent reaction partners for the synthesis of indazoles, however, they required a stoichiometric amount of copper(II) acetate for the C–N bond formation step. The transformation can be efficiently performed as a two step-one pot procedure to give a range of 1N-alkoxycarbonyl indazoles.


 Please wait while we load your content...
Please wait while we load your content...