Facile preparation of ZnxCd1−xS/ZnS heterostructures with enhanced photocatalytic hydrogen evolution under visible light
Abstract
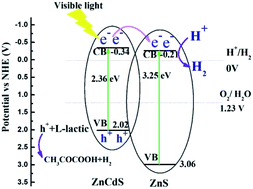
Hydrogen evolution from water using solar energy is regarded as a most promising process, thus, exploring efficient photocatalysts for water splitting is highly desirable. To avoid the rapid recombination of photogenerated electrons and holes in CdZnS semiconductors, ZnxCd1−xS/ZnS composites were synthesized via a one-step hydrothermal method and then annealed at 400 °C for 60 min under argon flow. ZnxCd1−xS/ZnS composites are composed of ZnS nanosheets decorated with ZnxCd1−xS nanorods, and TEM and UV-vis absorption spectra confirm the formation of the heterostructure between ZnxCd1−xS nanorods and ZnS nanosheets. Because of the well-matched band alignment, stronger optical absorption and larger carrier density, Zn0.2Cd0.8S/ZnS has the highest hydrogen production, with a photocatalytic hydrogen production rate up to 16.7 mmol g−1 h−1 under visible light irradiation. Moreover, the photocatalyst also exhibits high stability and good reusability for hydrogen production reaction. The facile and efficient approach for ZnS based heterostructures could be extended to other metal compound materials.


 Please wait while we load your content...
Please wait while we load your content...