High-pressure phase transition of AB3-type compounds: case of tellurium trioxide†
Abstract
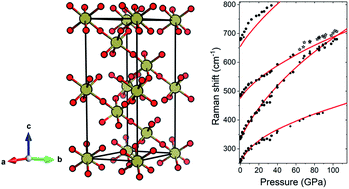
Tellurium trioxide, TeO3, is the only example of a trioxide adopting at ambient conditions the VF3-type structure (a distorted variant of the cubic ReO3 structure). Here we present a combined experimental (Raman scattering) and theoretical (DFT modelling) study on the influence of high pressure (exceeding 100 GPa) on the phase stability of this compound. In experiments the ambient-pressure VF3-type structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c symmetry) is preserved up to 110 GPa. In contrast, calculations indicate that above 66 GPa the R
c symmetry) is preserved up to 110 GPa. In contrast, calculations indicate that above 66 GPa the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c structure should transform to a YF3-type polymorph (Pnma symmetry) with the coordination number of Te6+ increasing from 6 to 8 upon the transition. The lack of this transition in the room-temperature experiment is most probably connected with energetic barriers, in analogy to what is found for compressed WO3. The YF3-type phase is predicted to be stable up to 220 GPa when it should transform to a novel structure of R
c structure should transform to a YF3-type polymorph (Pnma symmetry) with the coordination number of Te6+ increasing from 6 to 8 upon the transition. The lack of this transition in the room-temperature experiment is most probably connected with energetic barriers, in analogy to what is found for compressed WO3. The YF3-type phase is predicted to be stable up to 220 GPa when it should transform to a novel structure of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry and Z = 18. We analyse the influence of pressure on the band gap of TeO3, and discuss the present findings in the context of structural transformations of trioxides and trifluorides adopting an extended structure in the solid state.
symmetry and Z = 18. We analyse the influence of pressure on the band gap of TeO3, and discuss the present findings in the context of structural transformations of trioxides and trifluorides adopting an extended structure in the solid state.


 Please wait while we load your content...
Please wait while we load your content...