Synthesis of catalysts with fine platinum particles supported by high-surface-area activated carbons and optimization of their catalytic activities for polymer electrolyte fuel cells†
Abstract
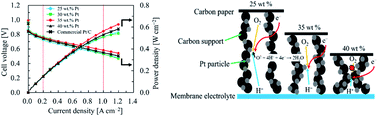
Herein, we demonstrated that carbon-supported platinum (Pt/C) is a low-cost and high-performance electrocatalyst for polymer electrolyte fuel cells (PEFCs). The ethanol reduction method was used to prepare the Pt/C catalyst, which was realized by an effective matching of the carbon support and optimization of the Pt content for preparing a membrane electrode assembly (MEA). For this, the synthesis of Pt/C catalysts with different Pt loadings was performed on two different carbons (KB1600 and KB800) as new support materials. Analysis of the XRD pattern and TEM images showed that the Pt nanoparticles (NPs) with an average diameter of ca. 1.5 nm were uniformly dispersed on the carbon surface. To further confirm the size of the NPs, the coordination numbers of Pt derived from X-ray absorption fine structure (XAFS) data were used. These results suggest that the NP size is almost identical, irrespective of Pt loading. Nitrogen adsorption–desorption analysis indicated the presence of mesopores in each carbon. The BET surface area was found to increase with increasing Pt loading, and the value of the BET surface area was as high as 1286 m2 gcarbon−1. However, after 40 wt% Pt loading on both carbons, the BET surface area was decreased due to pore blockage by Pt NPs. The oxidation reduction reaction (ORR) activity for Pt/KB1600, Pt/KB800 and commercial Pt/C was evaluated by Koutecky–Levich (K–L) analysis, and the results showed first-order kinetics with ORR. The favourable surface properties of carbon produced Pt NPs with increased density, uniformity and small size, which led to a higher electrochemical surface area (ECSA). The ECSA value of the 35 wt% Pt/KB1600 catalyst was 155.0 m2 gpt−1 higher than that of the Pt/KB800 and commercial Pt/C (36.7 wt%) catalysts. A Higher ECSA indicates more available active sites for catalyst particles. The single cell test with MEA revealed that the cell voltage in the high current density regions depends on the BET surface area, and the durability of the 35 wt% Pt/KB1600 catalyst was superior to that of the 30 wt% Pt/KB800 and commercial Pt/C (46.2 wt%) catalysts. This suggests that an optimal ratio of Pt to Pt/KB1600 catalyst provides adequate reaction sites and mass transport, which is crucial to the PEFC's high performance.


 Please wait while we load your content...
Please wait while we load your content...