H2O2 production on a carbon cathode loaded with a nickel carbonate catalyst and on an oxide photoanode without an external bias†
Abstract
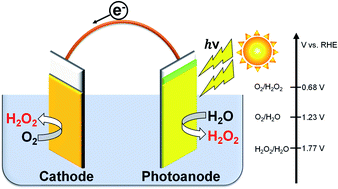
Efficient H2O2 production both on a carbon cathode modified with various metal salts and on an oxide photoanode was investigated. The cathodic current density and faradaic efficiency for H2O2 production (FE(H2O2)) on a carbon cathode in KHCO3 aqueous solution were significantly improved by the loading of an insoluble nickel carbonate basic hydrate catalyst. This electrode was prepared by a precipitation method of nickel nitrate and KHCO3 aqueous solution at ambient temperature. The nickel carbonate basic hydrate electrode was very stable, and the accumulated concentration of H2O2 was reached at 1.0 wt% at a passed charge of 2500C (the average FE(H2O2) was 80%). A simple photoelectrochemical system for H2O2 production from both the cathode and a BiVO4/WO3 photoanode was demonstrated without an external bias or an ion-exchange membrane in a one-compartment reactor under simulated solar light. The apparent FE(H2O2) from both electrodes was calculated to be 168% in total, and the production rate of H2O2 was approximately 0.92 μmol min−1 cm−2. The solar-to-chemical energy conversion efficiency for H2O2 production (STCH2O2) without an external bias was approximately 1.75%.


 Please wait while we load your content...
Please wait while we load your content...