Improved herbicide discovery using physico-chemical rules refined by antimalarial library screening†
Abstract
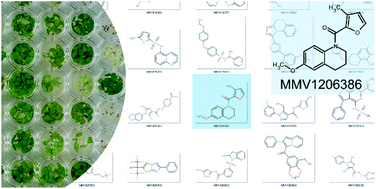
Herbicides have physico-chemical properties not unlike orally-delivered human drugs, but are known to diverge in their limits for proton donors, partition coefficients and molecular weight. To further refine rules specific for herbicides, we exploited the close evolutionary relationship between Plasmodium falciparum and plants by screening the entire Malaria Box, a chemical library of novel chemical scaffolds with activity against the blood stage of P. falciparum. Initial screening against Arabidopsis thaliana on agar media and subsequently on soil demonstrated the crucial nature of log P and formal charge are to active molecules. Using this information, a weighted scoring system was applied to a large chemical library of liver-stage effective antimalarial leads, and of the six top-scoring compounds, one had potency comparable to that of commercial herbicides. This novel compound, MMV1206386, has no close structural analogues among commercial herbicides. Physiological profiling suggested that MMV1206386 has a new mode of action and overall demonstrates how weighted rules can help during herbicide discovery programs.


 Please wait while we load your content...
Please wait while we load your content...