High pressure polymorphism of LiBH4 and of NaBH4†
Abstract
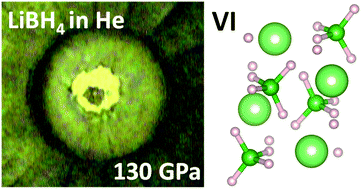
The pressure-induced structural changes in LiBH4 and in NaBH4 have been investigated experimentally up to 290 GPa by coupling Raman spectroscopy, infrared absorption spectroscopy and synchrotron X-ray diffraction. This data set is also analysed in the light of Density Functional Theory calculations performed up to 600 GPa. The [BH4]− unit appears to be remarkably resistant under pressure. NaBH4 remains stable in the known Pnma γ-phase up to 200 GPa and calculations predict a transition to a metallic polymeric C2/c phase at about 480 GPa. LiBH4 is confirmed to exhibit a richer polymorphism. A new Pnma orthorhombic phase VI is found to be stable above 60 GPa and there are hints of a possible phase VII above 160 GPa. DFT calculations predict that two other high pressure LiBH4 phases should appear at about 290 and 428 GPa. A very slight solubility of H2 inside phases II, III and V of LiBH4 is observed. A NaBH4(H2)0.5 complex is predicted to be stable above 150 GPa.


 Please wait while we load your content...
Please wait while we load your content...